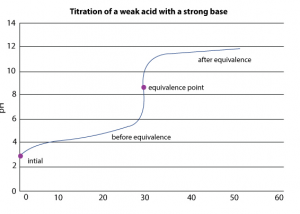
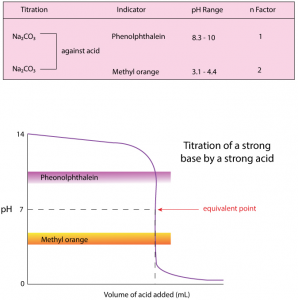
The analytical method wherein the concentration of a substance in a solution is estimated by adding exactly the same number of equivalents of another substance present in a solution of known concentration is called volumetric analysis.
This is the basic principle of titration. Another name for volumetric analysis is titrimetric analysis.
The volumetric analysis can be classified into three types:
- 1. Simple titration
- 2. Back titration
- 3. Double titrations
SIMPLE TITRATION
The main aim of simple titration is to find the concentration of an unknown solution with the aid of the known concentration of another solution. Simple titration can again be classified into four different types:
- Acid-base titrations
- Redox titrations
- Precipitation titrations and
- Complexometric titrations
Acid-base titrations
An acid-base indicator gives different colors of different compounds. An indicator is chosen in a particular titration based on the pH-range of the indicator and the pH change near the equivalence point.
Redox Titrations
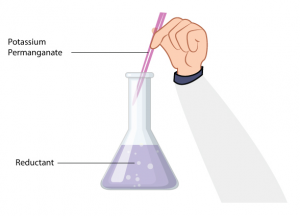 An oxidant can be estimated by adding reductant in redox titrations (or vice-versa). An example of this is; Fe2+ ions can be estimated by titration against acidified KMnO4 solution when Fe2+ ions are oxidized to Fe3+ ions and KMnO4 is reduced to Mn2+ in an acidic medium. Also, KMnO4 is a self-indicator. At the equivalence point, it can be noticed that it discharges a purple color.
An oxidant can be estimated by adding reductant in redox titrations (or vice-versa). An example of this is; Fe2+ ions can be estimated by titration against acidified KMnO4 solution when Fe2+ ions are oxidized to Fe3+ ions and KMnO4 is reduced to Mn2+ in an acidic medium. Also, KMnO4 is a self-indicator. At the equivalence point, it can be noticed that it discharges a purple color.Mn + 8H+ + 5e– → Mn2+ + 4H2O
Fe2+ → Fe3+ + e–] × 5
Mn + 8H+ + 5Fe2+ → Mn2+ + 5Fe3+ + 4H2O
(n=5) (n=1)
Acidified KCr2O7 can also be employed instead of KmnO4. Other redox titrations are iodimetry, iodometry etc.
I2 + 2Na2S2O3 → 2NaI + Na2S4O6
This is known to be an indirect method of estimation of iodine. Here, an oxidizing agent is made to react with an excess of solid KI. The oxidizing agent oxidizes I– to I2. The next step is to make the liberated I2 to react with Na2S2O3 solution.
Precipitation Titrations
In a titration of this form, a compound of very low solubility is formed by combining cations and anions. A solid residue can be separated out.
AgNO3 + NaCl → AgCI¯ (white) + NaNO3
BaCI2 + H2SO4 → BaSO4¯ (white) + 2HCI
CuSO4 + 4NH4OH → [Cu (NH3)4] SO4 + 4H2O
AgNO3 + 2KCN → K [Ag (CN) 2] + KNO3
Certain conditions have to be satisfied for back titrations to work:
- Compounds ‘A’, ‘B’ and ‘C’ which are provided should satisfy the condition that ‘A’ and ‘B’ react with each other.
- ‘A’ and pure ‘C’ should also react with each other but the impurity present in ‘C’ is not supposed to not react with ‘A’.
- Another important condition is that the product of ‘A’ and ‘C’ should not react with ‘B’.
Now, a certain volume of ‘A’ in a flask is taken out (the equivalents of ‘A’ taken has to be greater than equivalents of pure ‘C’ in the sample) and then a simple titration using ‘B’ is performed.
Na2CO3 + HCI → NaHCO3 + NaCI ……. (ii)
NaHCO3 + HCI → NaCI + CO2 + H2O …… (iii)
When HCI is added to the alkaline solution, alkali is neutralized. This results in the pH of the solution getting decreased. The pH decreases initially at a rapid pace since the strong base (NaOH) is neutralized completely. Whereas when Na2CO3 is converted to NaHCO3 completely, the solution remains weakly basic. This is due to the presence of NaHCO3 (which is weaker as compared to Na2CO3). It is at this point, where it is noticed that phenolphthalein changes color since it requires a weakly basic solution to show the color change.
Thus, in conclusion, phenolphthalein changes color when the solution contains weakly basic NaHCO3 along with other neutral substances while methyl orange changes color when the solution contains weakly acidic H2CO3 along with other neutral substances.
- a) To get accurate values, one must take special care to clean all the apparatus with distilled water. Even the slight presence of any other chemical will lead to mistakes in the result.
- b) While adding an acid/base, it should be done drop-wise.
- c) Contamination should be avoided as much as possible.
- d) While taking the readings, make sure that the markings are at your eye level.
- e) In the case of colorless solutions, the lower meniscus is read whereas the upper meniscus is read for colored solutions.
- f) For pipetting any solution, always use the index finger.
- g) Make sure that the burette is not leaking and that there are no air bubbles trapped inside it
- h) The indicator should never be used in excess.
- i) Take extra care to fill the pipette to get accurate readings.